Concurrent activation of striatal direct and indirect pathways during action initiation
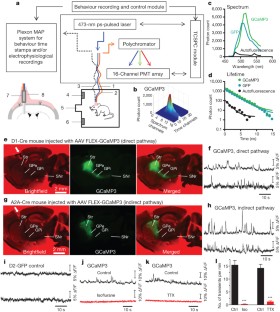
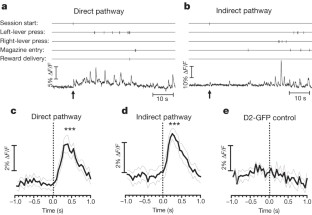
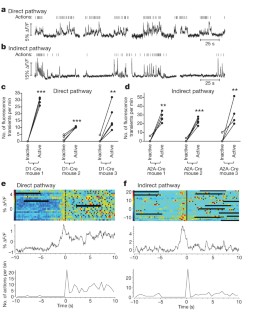
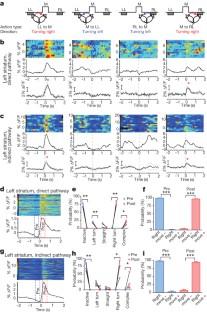
The basal ganglia are subcortical nuclei that control voluntary actions, and they are affected by a number of debilitating neurological disorders 1,2,3,4 . The prevailing model of basal ganglia function proposes that two orthogonal projection circuits originating from distinct populations of spiny projection neurons (SPNs) in the striatum 5,6 —the so-called direct and indirect pathways—have opposing effects on movement: activity of direct-pathway SPNs is thought to facilitate movement, whereas activity of indirect-pathway SPNs is presumed to inhibit movement 1,2 . This model has been difficult to test owing to the lack of methods to selectively measure the activity of direct- and indirect-pathway SPNs in freely moving animals. Here we develop a novel in vivo method to specifically measure direct- and indirect-pathway SPN activity, using Cre-dependent viral expression of the genetically encoded calcium indicator (GECI) GCaMP3 in the dorsal striatum of D1-Cre (direct-pathway-specific 6,7 ) and A2A-Cre (indirect-pathway-specific 8,9 ) mice 10 . Using fibre optics and time-correlated single-photon counting (TCSPC) in mice performing an operant task, we observed transient increases in neural activity in both direct- and indirect-pathway SPNs when animals initiated actions, but not when they were inactive. Concurrent activation of SPNs from both pathways in one hemisphere preceded the initiation of contraversive movements and predicted the occurrence of specific movements within 500 ms. These observations challenge the classical view of basal ganglia function and may have implications for understanding the origin of motor symptoms in basal ganglia disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
196,21 € per year
only 3,85 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
The cerebellum directly modulates the substantia nigra dopaminergic activity
Article 25 January 2024
Action suppression reveals opponent parallel control via striatal circuits
Article 06 July 2022

Basal ganglia–spinal cord pathway that commands locomotor gait asymmetries in mice
Article Open access 12 February 2024
References
- Albin, R. L., Young, A. B. & Penney, J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci.12, 366–375 (1989) ArticleCASGoogle Scholar
- DeLong, M. R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci.13, 281–285 (1990) ArticleCASGoogle Scholar
- Mink, J. W. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch. Neurol.60, 1365–1368 (2003) ArticleGoogle Scholar
- Hikosaka, O., Takikawa, Y. & Kawagoe, R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev.80, 953–978 (2000) ArticleCASGoogle Scholar
- Alexander, G. E. & Crutcher, M. D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci.13, 266–271 (1990) ArticleCASGoogle Scholar
- Gerfen, C. R. & Surmeier, D. J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci.34, 441–466 (2011) ArticleCASGoogle Scholar
- Gerfen, C. R. et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science250, 1429–1432 (1990) ArticleADSCASGoogle Scholar
- Schiffmann, S. N., Jacobs, O. & Vanderhaeghen, J. J. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J. Neurochem.57, 1062–1067 (1991) ArticleCASGoogle Scholar
- Schiffmann, S. N., Fisone, G., Moresco, R., Cunha, R. A. & Ferre, S. Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol.83, 277–292 (2007) ArticleCASGoogle Scholar
- Gong, S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci.27, 9817–9823 (2007) ArticleCASGoogle Scholar
- Nambu, A. Seven problems on the basal ganglia. Curr. Opin. Neurobiol.18, 595–604 (2008) ArticleCASGoogle Scholar
- Brown, P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol.17, 656–664 (2007) ArticleCASGoogle Scholar
- Chan, C. S., Surmeier, D. J. & Yung, W. H. Striatal information signaling and integration in globus pallidus: timing matters. Neurosignals14, 281–289 (2005) ArticleCASGoogle Scholar
- Fuller, D. R., Hull, C. D. & Buchwald, N. A. Intracellular responses of caudate output neurons to orthodromic stimulation. Brain Res.96, 337–341 (1975) ArticleCASGoogle Scholar
- Parent, A. et al. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci.23, S20–S27 (2000) ArticleCASGoogle Scholar
- Lima, S. Q., Hromadka, T., Znamenskiy, P. & Zador, A. M. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE4, e6099 (2009) ArticleADSGoogle Scholar
- Zariwala, H. A. et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci.32, 3131–3141 (2012) ArticleCASGoogle Scholar
- Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods6, 875–881 (2009) ArticleCASGoogle Scholar
- Dombeck, D. A., Harvey, C. D., Tian, L., Looger, L. L. & Tank, D. W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nature Neurosci.13, 1433–1440 (2010) ArticleCASGoogle Scholar
- Kerr, J. N. & Denk, W. Imaging in vivo: watching the brain in action. Nature Rev. Neurosci.9, 195–205 (2008) ArticleCASGoogle Scholar
- Schnütgen, F. et al. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nature Biotechnol.21, 562–565 (2003) ArticleGoogle Scholar
- Hikosaka, O., Sakamoto, M. & Usui, S. Functional properties of monkey caudate neurons. II. Visual and auditory responses. J. Neurophysiol.61, 799–813 (1989) ArticleCASGoogle Scholar
- Jin, X. & Costa, R. M. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature466, 457–462 (2010) ArticleADSCASGoogle Scholar
- Samejima, K., Ueda, Y., Doya, K. & Kimura, M. Representation of action-specific reward values in the striatum. Science310, 1337–1340 (2005) ArticleADSCASGoogle Scholar
- Goldberg, J. H. & Fee, M. S. A cortical motor nucleus drives the basal ganglia-recipient thalamus in singing birds. Nature Neurosci.15, 620–627 (2012) ArticleCASGoogle Scholar
- Kravitz, A. V. et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature466, 622–626 (2010) ArticleADSCASGoogle Scholar
- Durieux, P. F. et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nature Neurosci.12, 393–395 (2009) ArticleCASGoogle Scholar
- Bateup, H. S. et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc. Natl Acad. Sci. USA107, 14845–14850 (2010) ArticleADSCASGoogle Scholar
- Elangovan, M., Day, R. N. & Periasamy, A. Nanosecond fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy to localize the protein interactions in a single living cell. J. Microsc.205, 3–14 (2002) ArticleMathSciNetCASGoogle Scholar
- Schweitzer, D. et al. Towards metabolic mapping of the human retina. Microsc. Res. Tech.70, 410–419 (2007) ArticleCASGoogle Scholar
- Lue, N. et al. Live cell refractometry using Hilbert phase microscopy and confocal reflectance microscopy. J. Phys. Chem. A113, 13327–13330 (2009) ArticleCASGoogle Scholar
- Binding, J. et al. Brain refractive index measured in vivo with high-NA defocus-corrected full-field OCT and consequences for two-photon microscopy. Opt. Express19, 4833–4847 (2011) ArticleADSCASGoogle Scholar
- Costa, R. M., Cohen, D. & Nicolelis, M. A. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr. Biol.14, 1124–1134 (2004) ArticleCASGoogle Scholar
- Perkins, K. L. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J. Neurosci. Methods154, 1–18 (2006) ArticleCASGoogle Scholar
Acknowledgements
We thank C. R. Gerfen for gifts of multiple bacterial artificial chromosome (BAC) transgenic mouse lines; L. L. Looger and the Howard Hughes Medical Institute (HHMI) for permission to use AAV GCaMP3 vectors and GCaMP3 mice; S. R. Ikeda for assistance with Ca 2+ imaging in brain slices; G. Luo for mouse genotyping; C. Thaler for assistance with FLIM curve analysis; B. Mathur and M. Davis for assistance with brain slice electrophysiology and histology; and A. Martin for assistance with AAV vector injection. This work was supported by the Division of Intramural Clinical and Biological Research of the NIAAA, European Research Council STG 243393, an International Early Career Scientist grant from the Howard Hughes Medical Institute to R.M.C., a National Research Foundation of Korea grant (2011-0029485, 2012-0004003) and Smart IT Convergence System Research Center (SIRC-2011-0031866) from the Korean government (MEST) to S.B.J., and by an Ellison Medical Foundation grant (AG-NS-0944-12) to X.J.
Author information
- Guohong Cui and Sang Beom Jun: These authors contributed equally to this work.
Authors and Affiliations
- Section on In Vivo Neural Function, Laboratory for Integrative Neuroscience, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, 5625 Fishers Lane, Bethesda, Maryland 20892-9412, USA, Guohong Cui, Xin Jin, Michael D. Pham, David M. Lovinger & Rui M. Costa
- Department of Electronics Engineering, Ewha Womans University, Seoul 120-750, Korea, Sang Beom Jun
- Molecular Neurobiology Laboratory, The Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, California 92037, USA, Xin Jin
- Section on Cellular Biophotonics, Laboratory for Molecular Physiology, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, 5625 Fishers Lane, Bethesda 20892-9412, Maryland, USA, Steven S. Vogel
- Section on Synaptic Pharmacology, Laboratory for Integrative Neuroscience, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, 5625 Fishers Lane, Bethesda, Maryland 20892-9412, USA, David M. Lovinger
- Champalimaud Neuroscience Programme at Instituto Gulbenkian de Ciência and Champalimaud Centre for the Unknown, Lisbon 1400-038, Portugal, Rui M. Costa
- Guohong Cui








